|
Research Promotion Foundation of Cyprus, (POST-DOC/0718/0084): Optimizing immunotherapy in triple-negative breast cancer by normalizing the tumor microenvironment. December 2019 - November 2021 Project details Fellow: Fotios Mpekris Principal Investigator: Triantafyllos Stylianopoulos Total Funding: EUR €159,924 Funding scheme: DIDAKTOR (POST DOCTORAL RESEARCHERS), Research Innovation and Foundation Coordinated in: Cyprus (University of Cyprus) From 01/12/2019 to 30/11/2021 SUMMARY Inefficient delivery of cellular and molecular medicines to solid tumors can reduce dramatically the efficacy of treatment and thus, affect negatively the quality of life and survival of cancer patients. This can explain in large part why standard chemo- or immuno-therapies many times fail to treat specific cancer types, even though these therapies are potent enough to eradicate cancer cells in an in vitro system. Effective delivery of medicines to solid tumors is hindered by abnormalities in the structure of the tumor vasculature, which can reduce drastically tumor perfusion and as a result the systemic delivery of the medicine. In many tumor types blood vessels are hyper-permeable, leaving large interendothelial openings, which causes fluid loss from the vascular to the interstitial space of the tumor. Additionally, because of rapid cancer cell proliferation in the confined space of the tumor, cancer cells exert mechanical forces on blood vessels, causing vessel compression. Both vessel hyper-permeability and compression can reduce tumor blood flow, rendering tumors hypo-perfused and hypoxic. Impaired blood supply and hypoxia help cancer cells evade the immune system and increase their invasive and metastatic potential. Particularly, hypo-perfusion can reduce the number of immune cells that infiltrate into the tumor, while hypoxia renders tumor microenvironment immunosuppressive and attenuates the killing potential of effector immune cells. The hypothesis of the proposed research is that combined treatment of cancers that have both hyper-permeable and compressed vessels - such as a subset of breast tumors - by repurposing common anti-angiogenic and anti-fibrotic drugs and employing an immunotherapeutic agent can optimize intratumoral blood vessel functionality and thus, the efficacy of immunotherapy. To test our hypothesis, we propose to carry out in vivo studies in two orthotopic triple negative breast tumor models and employ an anti-angiogenic agent, repurpose a clinically approved anti-fibrotic drug and employ an immune checkpoint inhibitor antibody as the immunotherapy agent. The specific research objectives of the proposal are: Research Objective 1: Test the ability of the combined to optimize perfusion and enhance the efficacy of immunotherapy Research Objective 2: Test the ability of the combined strategy to improve perfusion and immunotherapy treatment of breast cancer metastasis. Research Objective 3: Test the ability of the combined strategy to enhance the efficacy of the treatment and increase overall survival of mice. RESULTS (A) Combining microenvironment normalization strategies to improve cancer immunotherapy We developed a mathematical framework [1] to determine how tumor microenvironment normalization strategies—specifically, vascular and stroma normalization— might improve immunotherapy efficacy and provide guidelines for designing effective combinatorial therapeutic strategies for the experimental studies. Based on our previous study [2] we developed a continuum mathematical modeling framework to account for interactions among different types of cancer cells, immune cells, tumor blood vessels, oxygen supply and drug delivery. Furthermore, we take into account the dependence of the functional vasculature on stroma components, the concentration gradients of several proangiogenic molecules (Figure 1).
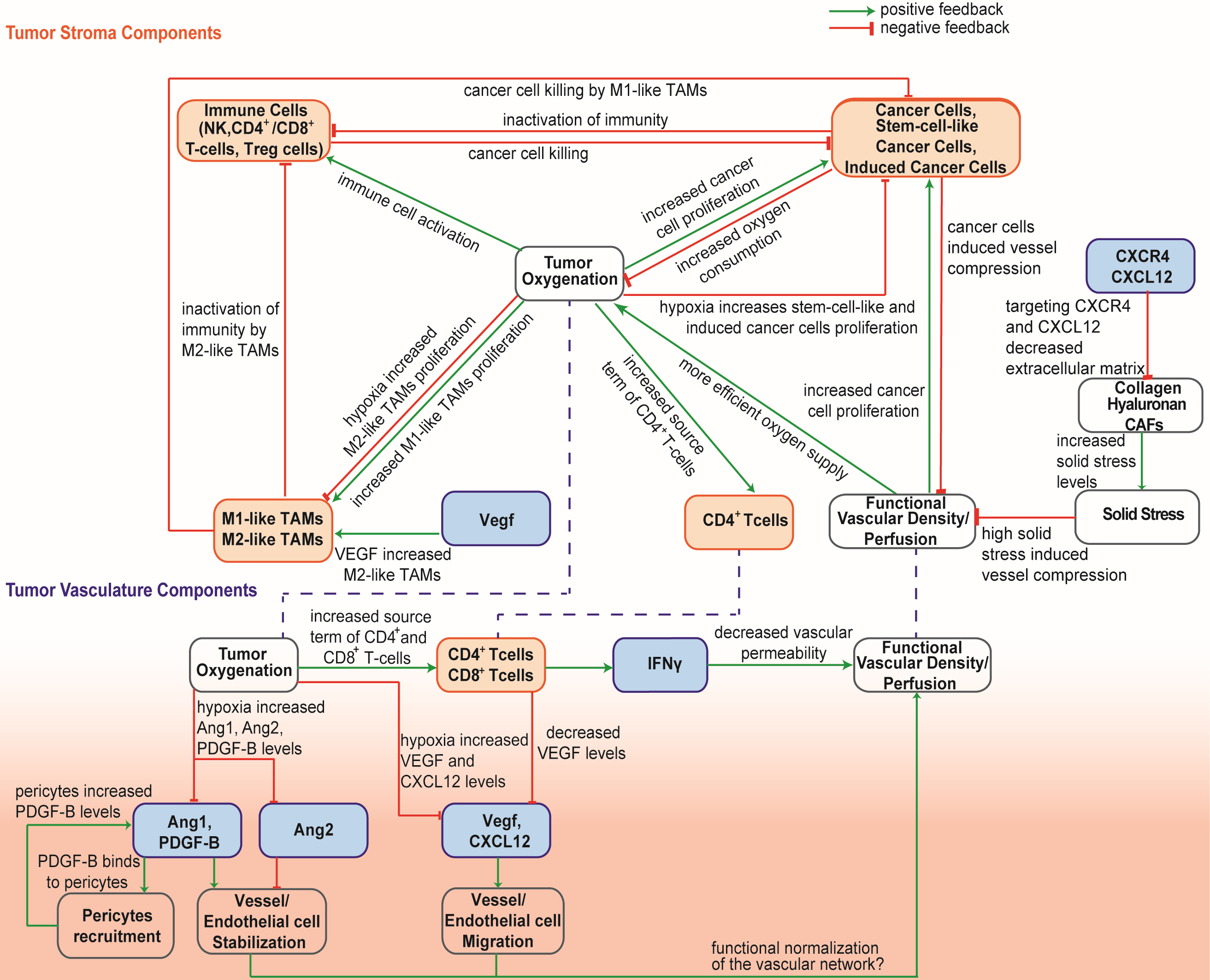
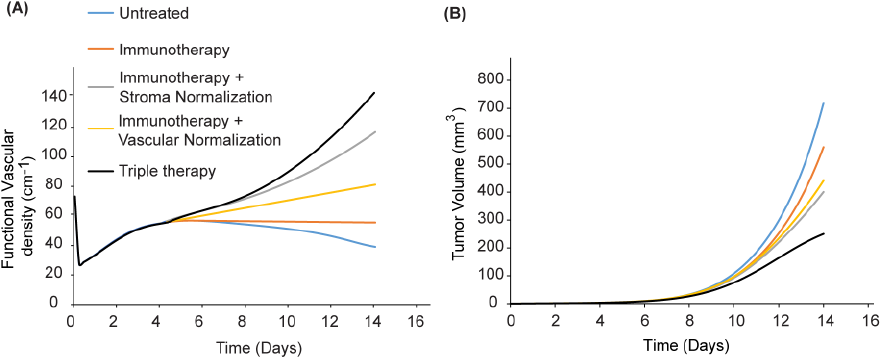
Figure 1: Schematic of the interactions among model components. We investigated the effect of a triple therapy combining anti-angiogenic, stroma-normalizing and immune therapy. Given that stroma normalization decompresses tumor blood vessels, it could increase the number of perfused vessels that anti-angiogenic therapy could normalize. Therefore, it is reasonable to hypothesize that combined normalization strategies would further improve perfusion. Indeed, our model predicts that combination of the two strategies with immunotherapy improves functional vascular density and tumor growth delay (Figure 2). Figure 2: Effect of simultaneous triple therapy of vascular and stroma normalization combined with immunotherapy on tumor volume. Triple therapy is more effective compared to the combinatorial treatment of immunotherapy with vascular normalization or stroma normalization.
References
Exploitation and dissemination Conferences/forums and public engagement activities:
Journal Paper Publications (Peer-reviewed) |