|
ImmunoMECH Horizon 2020 - Marie Skłodowska-Curie Individual Fellowship (WF-01-2018-867455 Widening Fellowships, ImmunoMECH): High-performance biomechanical model of combined immunotherapy and antiangiogenic cancer treatment. June 2019 - May 2021; €145,941.
Project details Fellow: Odysseas Kokkinos Summary Inefficient delivery of cellular and molecular medicines to solid tumors can reduce dramatically the efficacy of treatment and affect negatively the quality of life and survival of cancer patients. This explains in large part why standard chemo- or immuno-therapies often fail to treat specific cancer types, even though these therapies are potent to eradicate cancer cells in an in vitro system. Abnormalities in the structure of the tumor vasculature hinder tumor perfusion and as a result the systemic delivery of the medicine. In many tumor types blood vessels are hyper-permeable, leaving large interendothelial openings, which causes fluid loss from the vascular to the interstitial tumor space. Additionally, vessel compression is induced through rapid cancer cell proliferation in the confined space of the tumor. Both vessel hyper-permeability and compression reduce tumor blood flow, rendering tumors hypo-perfused and hypoxic. Impaired blood supply and hypoxia help cancer cells evade the immune system and increase their invasive and metastatic potential. Normalization of the tumor vasculature is a clinical strategy to repair abnormalities of the tumor vessels in order to improve perfusion, oxygenation and delivery of medicines. It is based on the use of anti-angiogenic agents exhibiting various degrees of success. Immunotherapy is gaining interest as an effective therapeutic approach against cancer, while it has been recently suggested to induce more robust vessel normalization. The objective of the proposed research is the optimal design of the combined (immunotherapy and anti-angiogenic treatment) therapeutic approach in order to explore the intrinsic mechanisms of the procedures described above. To this end, a dual numerical and experimental study is proposed, in order to model and experimentally validate the vascular normalization procedure, focusing on the combined effect of anti-angiogenic and immunotherapeutic agents in the generic case of a tumor perfusion and evolution system. Serving this purpose, a synergy of efficient stochastic simulation approaches based on efficient data driven models incorporating machine learning approaches, innovative numerical solution methods, advanced optimization algorithms and recent advances in high performance computing technology will be employed.
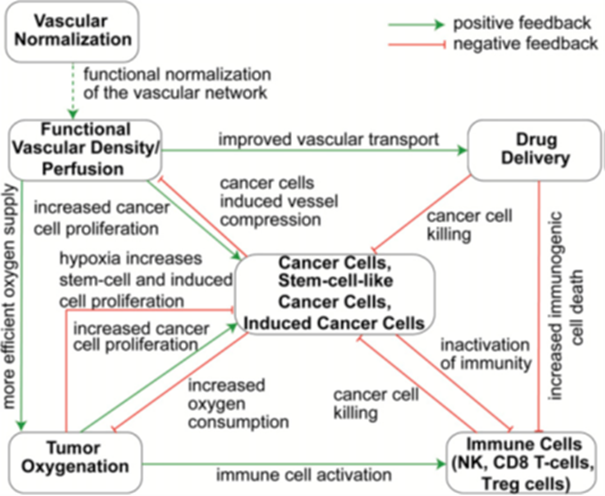
Fig1. Schematic of the existing model, which will be extended for TAMs, anti-angiogenic drugs and immunotherapeutics.
Overview of the project Studies have shown that tumor cells are able to evade immune responses by activating negative regulatory pathways, also known as immune checkpoints, that block T-cell activation[1,2]. Despite the promise of immunotherapy, the absence of dramatic immunotherapeutic responses has been attributed to a variety of factors, including hindering of immune cell delivery and activity in the tumor owed to the hypo-perfused tumor microenvironment (TME)[2,3]. Perfusion rates in some regions of a tumor can be significantly lower than that in the peri-tumor normal tissue, leading to inadequate delivery of medicines, which in turn, compromises the efficacy of cancer therapies, including immunotherapy. Particularly, hypo-perfusion can reduce the number of immune cells that infiltrate into the tumor, while hypoxia renders TME immunosuppressive and attenuates the killing potential of immune cells[4]. In many tumor types, impaired perfusion is owed in large part to the hyper-permeability of the tumor blood vessels that causes excessive fluid loss from the vasculature to the interstitial space of the tumor, and the chaotic structure of the vessel network that increases resistance to flow [5]. These two abnormalities can be restored using judicious doses of anti-angiogenic agents, which increases pericyte coverage and fortifies the leaky vessels without excessive pruning of vessels. This strategy – referred to as "vascular normalization" – can improve tumor perfusion and thereby increase oxygenation and delivery of medicines leading to improved treatment efficacy (Figure)[6]. However, normalization is dose- and time-dependent and thus, high doses or prolonged use of the anti-angiogenic drug can prune the vessels and reduce drastically perfusion[7]. Vascular normalization benefits immune response by improving perfusion and oxygenation skewing Tumor Associate Macrophage (TAM) polarization away from the immunosuppressive M2-phenotype, to M1-phenotype which is tumoricidal[11]. In turn, this results in activation of dendritic cells, cytotoxic T lymphocytes, and natural killer (NK) cells. Also, CBL found[9] that improving tumor perfusion via vessel normalization improves the function of immune cells, contributing to increased killing of cancer cells, also including killing of the more resistant stem-cell-like cancer cells. The main challenge now is to better understand how improving vascular normalization can enhance immunotherapy[10] in order to increase treatment efficiency. The proposal aims to provide insight into this mechanism. The challenging and innovative objective of ImmunoMECH is to develop a high-performance, large-scale stochastic tumor growth model in realistic geometries for the optimal design of the combined (immunotherapy and anti-angiogenic treatment) therapeutic approach, while simultaneously shedding light at the underlying mechanisms of the procedure described above. To this end, a dual numerical and experimental study is proposed, in order to model and experimentally validate the vascular normalization procedure, focusing on the combined effect of anti-angiogenic and immunotherapeutic agents in which the generic case of a tumor perfusion system and tumor evolution will be thoroughly modeled. To meet the aforementioned target, a novel robust computational model will be developed, in order to analyze and subsequently design therapies that will lead to maximum tumor regression. To handle the computational demands of the proposed research, a synergy of highly efficient stochastic simulation approaches based on efficient data driven models incorporating uptodate machine learning approaches (i.e., deep artificial neural networks (ΑΝΝ)), innovative numerical solution methods, advanced optimization algorithms and the exploitation of the recent advances in high performance computing (HPC) technology will be employed[11,12]. The achievements of this project are expected to vastly improve the design and modeling of cancer therapy beyond the current state of the art. Research Objectives Research Objective 1: Development of innovative data driven algorithms leading to realistic biomechanical modeling for immune cell/anti-angiogenic agents and tumor evolution that will harness the power of HPC environments comprised of distinct computing/architectural layers (CPUs, FPGA, GPUs, etc.). Research Objective 2: Experimental investigation of the vascular normalization/immunotherapy efficiency with in vivo experiments in animal tumor models. Research Objective 3: Experimental validation and verification of model parameters to optimize the therapeutic strategy in terms of immunotherapeutic and anti-angiogenic agents dosage. References [1]Pardoll D.M., Nature Reviews Cancer, 2012.
Journal Paper Publications “Reprogramming of mast cells restores T cell infiltration and sensitizes sarcomas to PD-L1 blockade”, paper under review “A high performance computational biomechanical model of tumor growth combining vasculature normalization and immunotherapy”, paper to be submitted “Characterization of optimal therapeutic schemes for combined anti-angiogenic and immunotherapeutic agents cancer therapy utilizing advanced HPC methodologies”, paper to be submitted
This project has received funding from the Horizon 2020 research and innovation programme under grant agreement 867455 |