Horizon 2020 - Marie Skłodowska-Curie Individual Fellowship (MSCA-IF-2014-658769 MYO-DESMOPLASIA): Modulating the behaviour of cancer myofibroblasts to control tumour desmoplasia. June 2015 - May 2017.
Project details
Fellow: Andreas Stylianou
Principal Investigator: Triantafyllos Stylianopoulos
Project ID: 658769
Total Funding: EUR 163 648,80
Topic(s): MSCA-IF-2014-EF - Marie Skłodowska-Curie Individual Fellowships (IF-EF)
Call for proposal: H2020-MSCA-IF-2014
Funding scheme: MSCA-IF-EF-ST - Standard EF
Coordinated in: Cyprus (University of Cyprus)
From 2015-06-01 to 2017-05-31
SUMMARY
In many tumors a desmoplastic reaction takes place during progression, which results in extensive production of collagen by stromal cells of the tumor, mainly fibroblasts and myofibroblasts. Tumor desmoplasia determines in large part the patho-physiology of solid tumors and poses a major barrier to effective drug delivery, affecting the overall survival of cancer patients. Here, the applicant proposes to test the hypothesis that the increase in extracellular matrix (ECM) stiffness and transforming growth factor-beta (TGFβ) activation often observed during tumor progression have additive effects on tumor desmoplasia. Therefore, targeting any of these parameters alone or in combination can reduce the desmoplastic response of the stromal cells. To explore this hypothesis, a combination of cutting-edge techniques will be employed. Specifically, a collagen ECM model, with pre-determined topography and tunable stiffness will be developed. Subsequently, fibroblasts and myofibroblasts will be cultured in the ECM models. Cells nanomechanical behavior and their morphodynamic alterations will be investigated with Atomic Force Microscopy and light/fluorescence microscopy under the presence or absence of TGFβ or anti-TGFβ agents. Finally, the effects of matrix stiffness along with different TGFβ concentrations in the expression pattern of genes encoding ECM components will be investigated using real-time PCR. The research results will elucidate the mechanisms of the interplay between matrix stiffness and TGFβ production in modulating the ability of fibroblasts and myofibroblasts to form tumor desmoplasia. In the proposed project, the fellow will acquire scientific and complementary skills according to his personalized career development plan and through advanced training, international and inter-sectoral mobility will reach a position of professional maturity in research.
Overview of the project
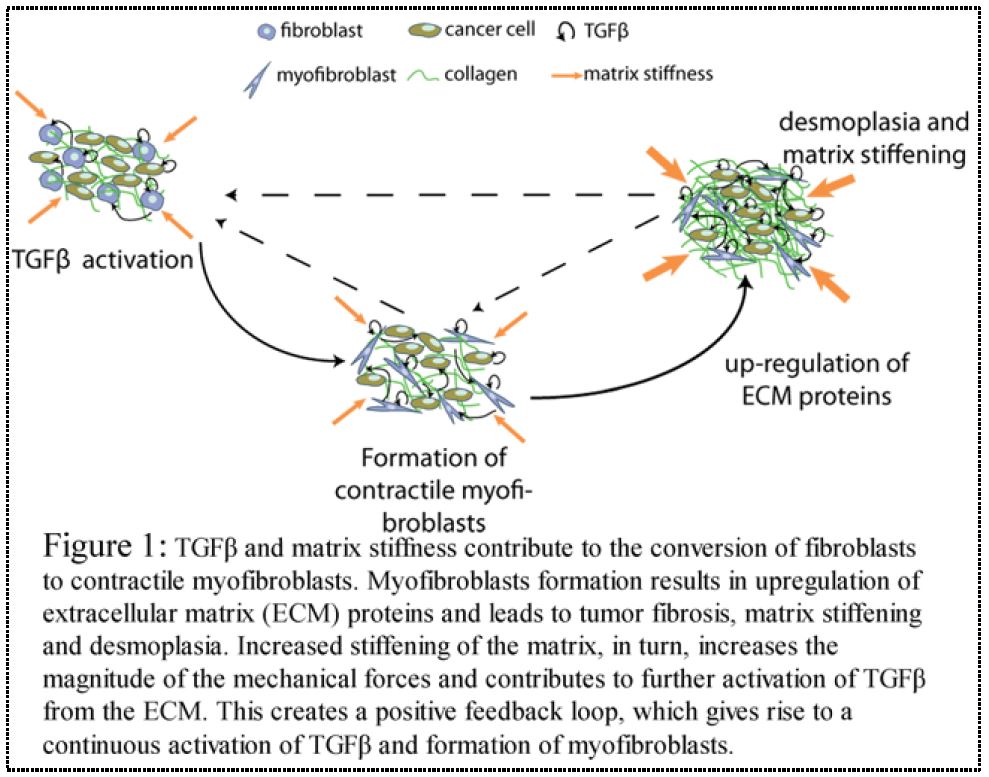
The tumor micro environment consists of the stromal cells (including fibroblasts and myofibroblasts, also known as cancer associated fibroblasts (CAFs) [1]), the extracellular matrix (ECM) and myriad soluble factors in the extracellular milieu whose importance in cancer progression and metastasis is indisputable [2]. In many types of tumors, including pancreatic, colon and breast cancers [3], the complex interplay among tumor micro environment components leads to remodeling and overproduction of the tumor ECM, resulting in a desmoplastic reaction. Desmoplasia is a cancer-specific type of fibrosis, characterized by presence of myofibroblasts and overproduction of ECM proteins, such as collagen type I [4], the major fibrous protein of the ECM. Desmoplasia stiffens the tumor tissue, and as a result, it increases the compressive mechanical forces in the interior of the tumor [5]. Although it has long been known that this fibrotic response inhibits drug delivery and enhance tumor progression and metastasis, the underlying mechanisms are still unclear [6]. Among the wide range of physical alterations that occur during cancer and the many TME constituents that are present, the myofibroblasts, the transforming growth factor beta (TGFβ) and the matrix stiffness - mainly due to collagen I over expression - stand out as key players, responsible for tumor desmoplasia. One pathway through which extensive ECM synthesis and remodeling occurs, is the activation of TGFβ [7] along with the mechanical forces exerted on fibroblasts from the ECM and other structural components of the tumor. Specifically, it is known that TGFβ activation and stiffening of the ECM contribute to the conversion of fibroblasts to contractile myofibroblasts (Fig. 1). Subsequently, myofibroblasts increase the synthesis of ECM proteins, such as collagens, while TGFβ down-regulates the expression of matrix-depleting metalloproteinases (MMPs), such as MMP-2 and MMP-9. Furthermore, TGFβ regulates the production of matrix-modifying enzymes, which increase the degree of collagen crosslinking [8]. This mechanism results in desmoplasia and further stiffening of the matrix. Matrix stiffening, in turn, will cause an increase in TGFβ expression and will further fibroblasts conversion [8]. Therefore, it appears to be a positive feedback loop, which gives rise to continuous activation of TGFβ and formation of myofibroblasts that exacerbate tumor desmoplasia [9] (Fig. 1). Taking all together, the hypothesis of the current proposal is that: matrix stiffness and TGFβ activation have additive effects on tumor desmoplasia. However, the underlying mechanisms, leading to the desmoplastic reaction of solid tumors are not yet fully understood. Elucidation of the role of matrix stiffness and TGFβ in controlling tumor desmoplasia can lead to new approaches for treating cancer. Specifically, it has been shown that targeting tumor desmoplasia can improve the systemic delivery of drugs and hinder metastasis [10].
Research Objective
The specific research objectives of the project are: .
Research Objective 1 (RO1): Develop of a collagen based ECM model, with pre-determined topography and tuneable stiffness.
Research Objective 2 (RO2): Characterize the mechanical properties and behaviour of fibroblasts and myofibroblasts cultured in the ECM models of RO1 as a function of TGFβ levels.
Research Objective 3 (RO3): Analyze gene expression of fibroblasts and myofibroblasts cultured in the matrix models of RO1 to indentify genes responseble for ECM production as a function of TGFβ levels and correlate to cell mechanical properties.
References
[1] G.S. Karagiannis, et al., Mol Cancer Res, 10 (2012) 1403-1418
[2] A.C. Shieh, Ann Biomed Eng, 39 (2011) 1379-1389.
[3] Z. Gang, et al., Microsc Res Techniq, 72 (2009) 672-678, G. Zhao, et al., J Surg Oncol, 102 (2010) 482-489.
[4] D. Hanahan, R.A. Weinberg, Cell, 144 (2011) 646-674.
[5] T. Stylianopoulos, et al., PNAS, 109 (2012) 15101-15108.
[6] T.R. Cox et al., Cancer Res, 73 (2013) 1721-1732.
[7] R.K. Jain, J.D. Martin, T. Stylianopoulos, Ann Rev Biomed Eng, (2014) 321-346.
[8] M. Egeblad, et al., Curr Opin Cell Biol, 22 (2010) 697-706.
[9] R.K. Jain, et al., Ann Rev Biomed Eng, (2014), 321-346, P. Papageorgis, T. Stylianopoulos, Int J Oncol, 46 (2015) 933-943.
[10] T. Stylianopoulos, et al.,PNAS, 109 (2012) 15101-15108, T. Stylianopoulos, R.K. Jain, PNAS, 110 (2013) 18632-18637.
Conclusions
In MYO-DESMOPLASIA Atomic Force Microscopy (AFM), fluorescence microscopy and image processing techniques were used to investigate the effect of TGF-β and collagen-induced stiffness on pancreatic fibroblasts and CAFs with regards to several cellular morphodynamic characteristics. More specifically, alterations in cell shape, cell spreading and stress fibers orientation were assessed, in the presence or absence of TGF-β, in three different collagen stiffness conditions. Furthermore, real-time PCR was employed to evaluate the expression of specific genes, such as Rac, in the presence and absence of TGF-β and under the influence of different collagen-stiffness conditions. Our results show that TGF-β and collagen stiffness significantly affect CAFs basic morphodynamic characteristics, such as cell elongation, cell spreading, and stress fiber orientation, while this was not the case for normal fibroblasts. Moreover, a significant correlation was revealed between cell spreading and Rac expression in both cell lines. Although more research is needed to elucidate the exact involvement of TGF-β and ECM stiffness in desmoplasia, these findings provide new insights that need to be taken into consideration for understanding of desmoplasia or even for the development of novel therapeutic approaches for treating cancer having TGF-β as a target molecule
Exploitation and dissemination
Conferences/forums and public engagement activities
· Dr. A. Stylianou participated in the EUROAFMForum 2016, June, 22-24, 2016, Geneva, Switzerland . The oral presentation was entitled "Effects of Transforming Growth Factor β on Pancreatic Cancer Associated Fibroblasts and Fibroblasts".
· The research was also presented to the public during the Researcher Night (30 September 2016), organized by the Research Promotion Foundation of Cyprus. Researcher Night is an event held to bring research and innovation to the forefront of media attention and emphasize its potential significance in the Cyprus economy. During the event audiences of different ages will have the opportunity to meet up with Cypriot researchers and see their work, in a festive, friendly atmosphere. A poster was presented by Dr. Stylianou with title: " Investigation of the effect of Tumor Growth Factor-β on Pancreatic Normal and Cancer Associated Fibroblasts".
· Dr. A. Stylianou also participated at the 14th Mediterranean Conference on Medical and Biological Engineering and Computing, MEDICON 2016; Paphos; Cyprus; 31 March 2016 through 2 April 2016.
Journal Paper Publications (Peer-reviewed)
· Stylianou A., V. Gkretsi, M. Louca and T. Stylianopoulos. Matrix stiffness modulates cytoskeleton remodeling and invasion of pancreatic fibroblasts. (Under review).
· Stylianou A., V. Gkretsi, and T. Stylianopoulos. Atomic Force Microscopy Nano-Characterization of 3D Collagen Gels with Tunable Stiffness. MethodsX 5:503-513 [DOI: 10.1016/j.mex.2018.05.009]
· Stylianou A., V. Gkretsi and T. Stylianopoulos. Transforming Growth Factor-β modulates Pancreatic Cancer Associated Fibroblasts cell shape, stiffness and invasion. BBA 1862(7):1537-1546 [DOI: 10.1016/j.bbagen.2018.02.009].
· Stylianou, A. (2017) "Atomic Force Microscopy for Collagen-Based Nanobiomaterial", Journal of Nanomaterials, art. id. 9234627 [DOI: 10.1155/2017/9234627]
· Kontomaris, S.V and Stylianou, A. (2017) "Atomic Force Microscopy for University Students, Application in Biomaterials" European Journal of Physics, 38 (3) [DOI: 10.1088/1361-6404/aa5cd6]
· Stylianou, A. and Stylianopoulos, T. (2016) "Atomic Force Microscopy Probing of Cancer Cells and Tumor Microenvironment Components" BioNanoScience,6 (1), 33-46 [DOI: 10.1007/s12668-015-0187-4]
· Gkretsi, V., Stylianou, A., Papageorgios, P., Polydorou, C. and Stylianopoulos, T. (2015) "Remodeling components of the tumor microenvironment to enhance cancer therapy" Frontiers in Oncology, 5 (214) [DOI: 10.3389/fonc.2015.00214].
Book chapter
· Stylianou, A., Gkretsi, V, Patrickios, C. and Stylianopoulos, T. (2017) "Chapter 29: Exploring the nano-surface of fibrotic tissues with AFM" In: Fibrosis: Methods and Protocols (Rittié L, ed), Springer-Verlag, Berlin-Heidelberg-New York, ISBN 978-1-4939-7112-1 [DOI: 10.1007/978-1-4939-7113-8_29].