Horizon 2020 - Marie Skłodowska-Curie Individual Fellowship (MSCA-IF-2014-657139 STROMAMECH): Targeting stromal cells to modify tumor mechanical microenvironment and optimize drug delivery. May 2015 - April 2017
SUMMARY
Current chemotherapeutic agents are potent enough to kill cancer cells. Nonetheless, failure of standard chemotherapies for many cancer types (e.g., pancreatic and breast cancers) is primarily attributed to these agents never reaching cancer cells in amounts sufficient for complete cure. In solid tumours, blood vessels are often compressed, drastically reducing perfusion, thus resulting in insufficient drug delivery. Vessel compression is a consequence of mechanical stresses accumulated within the tumour during progression. Alleviation of these stresses has the potential to reopen compressed vessels and improve tumour perfusion. Here, the applicant proposes to test the hypothesis that judicious depletion of stromal cells, namely the cancer-associated fibroblasts (CAFs), has the potential to alleviate stress levels in highly desmoplastic and hypoperfused tumours and, thus, enhance chemotherapy. To explore this hypothesis, a combination of cutting-edge computational and experimental techniques will be employed. Specifically, a structure-based biomechanical model will be developed to analyse the contribution of CAFs to the generation and transmission of forces within a tumour. Subsequently, in vivo studies will be performed in mice to validate model predictions and identify the degree of CAF depletion that optimizes the efficacy of treatment. Successful completion of the proposed research will reveal the role of CAFs on the biomechanical behaviour of tumours and contribute to developing a therapeutic strategy for treatment of hypovascular tumours. Therefore, the proposal negotiates a subject of considerable importance for European society and beyond. Furthermore, the proposed research and training will establish a bidirectional transfer of skills where the applicant's expertise in cell mechanics will be complemented by the Host's in cancer biophysics, thus enhancing the applicant's scientific potential and professional maturity, and promoting European research.
Research Objective
Research Objective 1. Development of a structure-based, biomechanical model for tumour growth focusing on the contribution of CAFs.
Research Objective 2. Execution of in vivo experiments on tumours grown in mice in order to:
a) validate model predictions and assess the accuracy of the model;
b) optimize combined treatment of chemotherapy with a CAF-depleting agent.
a) validate model predictions and assess the accuracy of the model;
b) optimize combined treatment of chemotherapy with a CAF-depleting agent.
Overview of the project
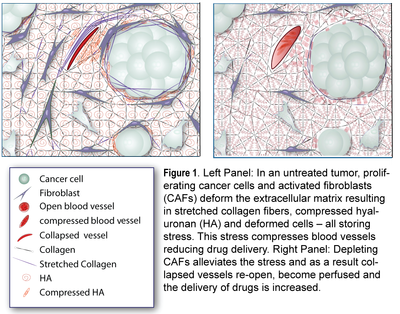
Previous studies have employed inhibitors of the Sonic Hedgehog (SHH) signaling pathway to achieve pharmacologic depletion of CAFs in pancreatic cancer animal models. CAFs depletion was shown to reduce number of CAFs and improve blood vessel functionality and the efficacy of chemotherapy. Despite these encouraging data, however phase-II clinical trials failed to show any benefit. However, these results could be due to different reasons, such as intrinsic resistance to gemcitabine as increased delivery of a drug might not benefit patients if cancer cells are or become resistant to that drug. Additionally, a series of recent in vivo studies have shown that deletion of CAFs by genetic manipulation in mouse models induces immunosuppression and promotes tumour progression in pancreatic cancers. Therefore manipulation of CAFs has been shown to both promote and restrain tumor progression, but any comparison between genetic deletion and pharmacologic depletion should be viewed with caution and take into account that these methods significantly differ from each other as genetic deletion is chronic and effects of genetic deletion are not reversible. On the other hand, pharmacologic depletion is acute and effects are reversible when the treatment stops.
In STROMAMECH, we revisited the use of SHH inhibitors to target CAFs with the aim to i) elucidate the mechanism of how CAFs depletion improves drug delivery, ii) extent and evaluate the potential use of SHH inhibitors to breast cancers, and iii) investigate whether SHH inhibition can improve not only chemotherapy, but also the efficacy of the most commonly used breast cancer nanomedicines, namely Abraxane® and Doxil®. To achieve our aims, we employed vismodegib (Erivedge®) in mouse tumor models for pancreatic and breast cancers to explore its ability to normalize the tumor microenvironment, decrease solid stress levels and improve tumor perfusion and therapeutic outcomes.
To elucidate the mechanism by which depletion of CAFs improves drug delivery, we initially hypothesized that depleting stromal cells in primary pancreatic tumors will reduce solid stresses and improved the functionality of tumor blood vessels. To investigate this, we employed two human pancreatic cancer cell lines, namely MiaPaCa2 and BxPC3 to develop xenograft tumor models in immunodeficient mice. We showed that vismodegib reduces solid stresses, decreases interstitial fluid pressure (IFP), improves perfusion, increases delivery of chemotherapy and improves therapeutic outcomes. Furthermore, we showed that these observations are not only due to the reduction in the activity of CAFs but also due to the decrease in collagen and hyaluronan tumor content due to SHH signaling pathway inhibition and downregulation of downstream key effector genes Gli1 and Gli2. Development of a structure-based biomechanical model agreed well with the experimental findings. Finally, we developed an orthotopic xenograft breast tumor model, using the human breast cancer cell line MCF10CA1a, to study the effect of combining vismodegib with two clinically approved nanoparticles of different sizes, Abraxane (10 nm) and Doxil (100 nm), and showed that vismodegib can also significantly enhance the efficacy of these common cancer nanomedicines.
References
[1] K.P. Olive, M.A. Jacobetz, C.J. Davidson, A. Gopinathan, D. McIntyre, D. Honess, B. Madhu, M.A. Goldgraben, M.E. Caldwell, D. Allard, K.K. Frese, G. Denicola, C. Feig, C. Combs, S.P. Winter, H. Ireland-Zecchini, S. Reichelt, W.J. Howat, A. Chang, M. Dhara, L. Wang, F. Ruckert, R. Grutzmann, C. Pilarsky, K. Izeradjene, S.R. Hingorani, P. Huang, S.E. Davies, W. Plunkett, M. Egorin, R.H. Hruban, N. Whitebread, K. McGovern, J. Adams, C. Iacobuzio-Donahue, J. Griffiths, D.A. Tuveson, Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer, Science, 324 (2009) 1457-1461.
[2] T. Stylianopoulos, J.D. Martin, V.P. Chauhan, S.R. Jain, B. Diop-Frimpong, N. Bardeesy, B.L. Smith, C.R. Ferrone, F.J. Hornicek, Y. Boucher, L.L. Munn, R.K. Jain, Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors, Proceedings of the National Academy of Sciences of the United States of America, 109 (2012) 15101-15108.
[3] B.C. Ozdemir, T. Pentcheva-Hoang, J.L. Carstens, X. Zheng, C.C. Wu, T.R. Simpson, H. Laklai, H. Sugimoto, C. Kahlert, S.V. Novitskiy, A. De Jesus-Acosta, P. Sharma, P. Heidari, U. Mahmood, L. Chin, H.L. Moses, V.M. Weaver, A. Maitra, J.P. Allison, V.S. LeBleu, R. Kalluri, Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival, Cancer cell, 25 (2014) 719-734.
[4] A.D. Rhim, P.E. Oberstein, D.H. Thomas, E.T. Mirek, C.F. Palermo, S.A. Sastra, E.N. Dekleva, T. Saunders, C.P. Becerra, I.W. Tattersall, C.B. Westphalen, J. Kitajewski, M.G. Fernandez-Barrena, M.E. Fernandez-Zapico, C. Iacobuzio-Donahue, K.P. Olive, B.Z. Stanger, Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma, Cancer cell, 25 (2014) 735-747.
[5] J.J. Lee, R.M. Perera, H. Wang, D.C. Wu, X.S. Liu, S. Han, J. Fitamant, P.D. Jones, K.S. Ghanta, S. Kawano, J.M. Nagle, V. Deshpande, Y. Boucher, T. Kato, J.K. Chen, J.K. Willmann, N. Bardeesy, P.A. Beachy, Stromal response to Hedgehog signaling restrains pancreatic cancer progression, Proceedings of the National Academy of Sciences of the United States of America, 111 (2014) E3091-3100.
Conclusions
In STROMAMECH, we employed vismodegib (Erivedge®) to inhibit the sonic-hedgehog pathway with the aim to i) elucidate the mechanism of how CAFs depletion improves drug delivery, ii) extent and evaluate the potential use of sonic-hedgehog inhibitors to breast cancers, and iii) investigate whether sonic-hedgehog inhibition improves not only chemotherapy, but also the efficacy of the most commonly used breast cancer nanomedicines, namely Abraxane® and Doxil®. We performed a series of in vivo experiments in animal tumor models and mathematical modeling and found that treatment with vismodegib normalizes the tumor microenvironment by reducing the proliferative CAFs and in cases the levels of collagen and hyaluronan. These modulations re-engineered the solid and fluid stresses in the tumors, improving blood vessel functionality. As a result, the delivery and efficacy of chemotherapy was improved in two models of pancreatic cancer. Additionally, vismodegib treatment significantly improved the efficacy of both Abraxane and Doxil in xenograft breast tumors. Our results suggest the use of vismodegib, and sonic hedgehog inhibitors in general, to enhance cancer chemo- and nanotherapy.This work is currently under review for publication.
Exploitation and dissemination
1. The mathematical model was presented at the scientific conference: ECCOMAS Congress 2016 5 - 10 JUNE 2016 Crete Island, Greece, European Congress on Computational Methods in Applied Sciences and Engineering The presentation was given by Dr. Athanasios Pirentis (The fellow) with title: "Contribution of cell-collagen fibre mechanical interplay to intratumoural solid stress build-up and implications for tumour growth"
2. The research was also presented to the public during the Research and Innovation Week, organized by the Research Promotion Foundation of Cyprus between November 24-28, 2016. Research and Innovation Week is an event held to bring research and innovation to the forefront of media attention and emphasize its potential significance in the Cyprus economy. A poster was presented by Dr. Pirentis with title: "Intratumoral component interactions and modification of the mechanical microenviornment to regulate solid stresses and optimize drug delivery in tumors".
3. Two research articles containing all the work performed during the period of the project is currently under preparation.
Progress beyond the state of the art.
In STROMAMECH, we elucidated the mechanism of how CAFs depletion improves drug delivery, ii) extended and evaluated the potential use of SHH inhibitors to breast cancers, and iii) proved that SHH inhibition can improve not only chemotherapy, but also the efficacy of the most commonly used breast cancer nanomedicines, namely Abraxane® and Doxil®.
Public awarness
Interview of Prof. Stylianopoulos for the Public Television of Cyprus (Link)
Interview of Prof. Stylianopoulos for the Public Radio of Cyprus (Link)